Updated December 2024
Is your team doing everything required to properly label hazardous products sold or used in Canada? The Hazardous Products Act (HPA) mandates that suppliers communicate those hazards through both product labels and Safety Data Sheets (SDS). The specific criteria your business must follow are laid out by the Hazardous Products Regulations (HPR).
The Latest Update to Canada’s Hazardous Products Regulations
If you’re currently responsible for hazardous product classification, labeling, or documentation, then you should know that the HPR has undergone some recent changes. Health Canada published amendments to the HPR on January 4, 2023, to begin the transition from the 5th edition of the GHS to the 7th edition, with some provisions from the 8th edition. These changes affect the HPA, HPR, and WHMIS.
What Is the GHS?
GHS stands for the Globally Harmonized System of Classification and Labelling of Chemicals. It is an international system that provides specific rules concerning chemical hazard classification, documentation, and labeling. GHS is used across many countries, serving as the foundation for WHMIS in Canada and OSHA’s HazCom in the United States.
The change means that many businesses will have to update their SDSs, as there are new information requirements and modifications to some classifications, including flammable gases, aerosols, and gases under pressure.
A variety of criteria that were once optional must now be included on all SDSs, including physical state, color, and more. Another change requires that hazardous ingredients in mixtures above cutoff levels must be disclosed even if they don’t contribute to the classification of the mixture.
A 3-year transition period has been put in place to allow suppliers, employers, and workers time to adjust to these changes, ending on December 14, 2025. During the transition period, businesses can choose to maintain compliance with either the former or the amended HPR but may not pick and choose provisions from both.
The Original Hazardous Products Regulations
The Government of Canada first implemented the HPR in 2015 and brought about the transition to the GHS standard for WHMIS 2015. The HPR replaced the older Controlled Products Regulations (CPR), which had provided the requirements for WHMIS 1988. This move came several years after the United States began its transition to GHS in 2012.
How to Protect Trade Secret Ingredients
One of the most significant concerns that businesses have when complying with the HPR is protecting their trade secrets or confidential business information (CBI). However, the HPR does allow for CBI protection on SDSs in some cases.
The CPR has previously laid out prescribed ranges for determining what CBI could be protected. In the initial publication of the HPR, Health Canada did not retain these prescribed concentration ranges for protection. Instead, if a chemical contributed to a health hazard, the actual concentration or the actual concentration range was mandated to be listed in Section 3 of the Safety Data Sheets (SDSs).
In fact, to protect the actual concentration or actual concentration ranges, a claim was required to be filed for CBI protection under the Hazardous Materials Information Review Act (HMIRA). The CBI protection needed to be used for any of the following with respect to Section 3:
- Mask the CAS registry number.
- Mask the chemical identity.
- Mask the chemical’s actual concentration or actual concentration range.
Once a CBI claim is validated, an HMIRA registry number (RN) is provided which needs to be displayed along with the granted or filed date.

Amendments made in 2017 remedied the CBI situation under the HPR. The amendment to the HPR indicates the allowance of prescribed concentration ranges to protect the actual concentration and actual concentration ranges without requiring filing with HMIRA.
What Are the HPR Concentration Ranges?
The following 13 prescribed concentration ranges have been defined and can be used for the purposes of protecting “trade secret” ingredients:
- (a) 0.1 to 1%
- (b) 0.5 to 1.5%
- (c) 1 to 5%
- (d) 3 to 7%
- (e) 5 to 10%
- (f) 7 to 13%
- (g) 10 to 30%
- (h) 15 to 40%
- (i) 30 to 60%
- (j) 45 to 70%
- (k) 60 to 80%
- (l) 65 to 85%
- (m) 80 to 100%
How to Use HPR Concentration Ranges
Below is a list of different scenarios that may be applicable to the use of prescribed concentration ranges on a Safety Data Sheet:
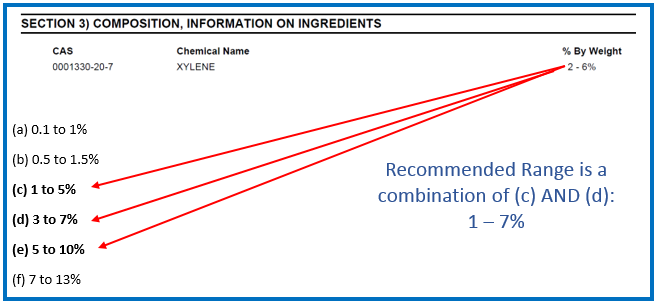
Scenario 1 – Using the Prescribed Concentration Ranges for the Actual Concentration of an Ingredient
If the actual concentration of an ingredient falls under more than one of the previously listed prescribed concentration ranges, then the selection of the range is at the discretion of the supplier or manufacturer.
Example for scenario 1:
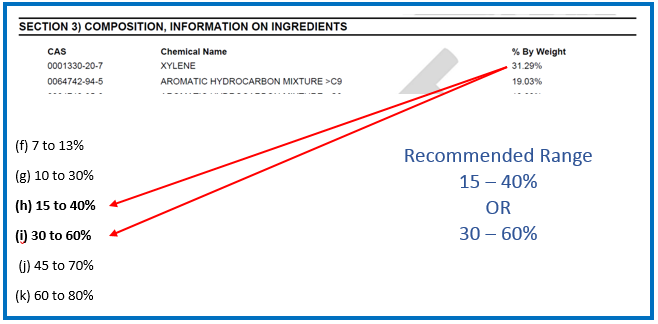
Scenario 2 – Using the Prescribed Concentration Ranges for the Actual Concentration Range of an ingredient
If the actual concentration range of an ingredient does not fall under one of the previously listed prescribed concentration ranges and it falls below 30%, then a customized combination of two consecutive ranges is allowed.
Example for scenario 2:
Maintaining Compliance With the HPR
It’s your organization’s responsibility to maintain compliance with the HPR in order to ensure workplace safety and avoid potential penalties. However, navigating hazard classification, CBI, and other details poses a significant challenge. The team at ERA is here to help you check all the boxes when it comes to SDSs, labels, and other documentation.
ERA’s SDS Authoring Software lets your team build complete safety data sheets compliant with GHS and a wide range of international standards. Ensure accuracy, compliance, and accessibility while saving your team time and money. If you’d like to find out more about our SDS Authoring Software, schedule a consultation with one of our project analysts today.
We also invite you to download our free GHS Hazard Classification Guide for more insight into this topic by clicking the button below.
This Blog was Co-Authored By:


May 4, 2018

Comments