Having compliant Safety Data Sheets (SDS) on hand is critical for all suppliers of substances and mixtures. Not only do they aid in effectively informing workers of all associated hazards, but they also help employers meet their obligation to assess health and safety risks from the presence of hazardous chemicals and to protect public health and the environment. The EU has slightly different rules than OSHA when it comes to SDS authoring, but all sheets must also comply with the Globally Harmonized System (GHS).
SDS are integral to the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulations. REACH requirements are everchanging and have continuously been adapted to include the Globally Harmonized System (GHS) rules. Further modifications have been made to these rules through amendments to Annex II of REACH. According to Regulation (EC) No 1272/2008 (Classification, Labelling and Packaging Regulation (CLP), SDS communicates safety information when a substance or mixture has been deemed hazardous. All SDS generated after 31 December 2022 must be formatted according to Regulation (EU) 2020/878; remember to adopt this new format as soon as possible when generating new SDS.
This article will list CLP SDS requirements and outline the changes made according to Regulation (EU) 2020/878.
A quick summary of Regulation 2020-878-EU Commission Updates
General Requirement
-
Use simple, clear, and precise language.
-
All SDS pages should be numbered or use phrases to indicate the presence of a following page (ex. Continued on next page or End of safety data sheet)
Section 1 Requirement
-
Indicate the Unique Formulation Identifier of all your mixtures in section 1 of your SDS.
-
The Product ID in Section 1 of SDS should indicate the word “Nanoform”, if any nanoforms are present.
Section 2 Requirement
-
Section 2.3 other hazards, should indicate the substance(s) with the concentration =>0,1 % by weight when included on the list established in accordance with Article 59(1) of REACH for having endocrine disrupting properties, or is identified as having endocrine disrupting properties in accordance with the criteria set out in Commission Delegated Regulation (EU) 2017/2100 or Commission Regulation (EU) 2018/605.
Other Requirements
-
Provide information on endocrine disruptors in sections 11 and 12.
CLP SDS Authoring Requirements
The EU has clearly outlined general requirements for SDS creation to address the included information's format and objectives which were briefly mentioned in the general section in the quick summary above. You must always take these into account before formulating your SDS. The first requirement states that must use clear and precise language, avoiding jargon or obscure acronyms. Avoid any language that may indicate that the substance is not hazardous, such as “may be dangerous”, “no health effects”, or “safe under most conditions of use” if they are inconsistent with the classification. As mentioned before, the second rule states that you must number all pages, to elaborate further the rule says you must specify the total on each page (for example, “page 1 of 10”), and indicate the presence of a following page (ex. Continued next page) and the end of the document (End of safety data sheet).
In addition to these necessary items, every safety data sheet must:
-
Be concise and prepared by a competent person (someone with sufficient knowledge for the completion of a section of SDS or the entire SDS such as a qualified chemist). Suppliers must always provide accurate product information.
-
Enable users to take protective measures in the workplace. The writer of the SDS must clearly inform the intended audience of the hazards of a substance or mixture and provide safe storage, handling, and disposal information.
-
List the information in such a way that allows employers to decipher if there are hazardous substances in the workplace and assess risk to the health and safety of workers during operating practices.
-
Avoid jargon that may be difficult for the reader to understand, as well as vague acronyms, abbreviations, or statements that may indicate that the product is not hazardous (if inconsistent with the product’s classification).
CLP Guidance on Completing the SDS
With the above considerations, you are ready to compile your SDS. Each SDS section addresses different properties and requires research, and even laboratory tests, to determine the appropriate classifications. Shown below are the sections of CLP SDS according to the EU.
SDS SECTION 1: Identification of the substance/mixture and of the company/undertaking
SDS SECTION 2: Hazards identification
SDS SECTION 3: Composition/information on ingredients
SDS SECTION 4: First aid measures
SDS SECTION 5: Firefighting measures
SDS SECTION 6: Accidental release measures
SDS SECTION 7: Handling and storage
SDS SECTION 8: Exposure controls/personal protection
SDS SECTION 9: Physical and chemical properties
SDS SECTION 10: Stability and reactivity
SDS SECTION 11: Toxicological information
SDS SECTION 12: Ecological information
SDS SECTION 13: Disposal considerations
SDS SECTION 14: Transport Information
SDS SECTION 15: Regulatory information
SDS SECTION 16: Other information
You may wonder which section to tackle first and how to compile them in a sequence that promotes efficient data collection. Fortunately, the EU provides the following framework to help you compile your SDS with ease. Sticking to the framework’s sequence (shown below) is highly recommended; identifying hazards in Section 2, for instance, may only be possible once you have determined the inputs of all other sections.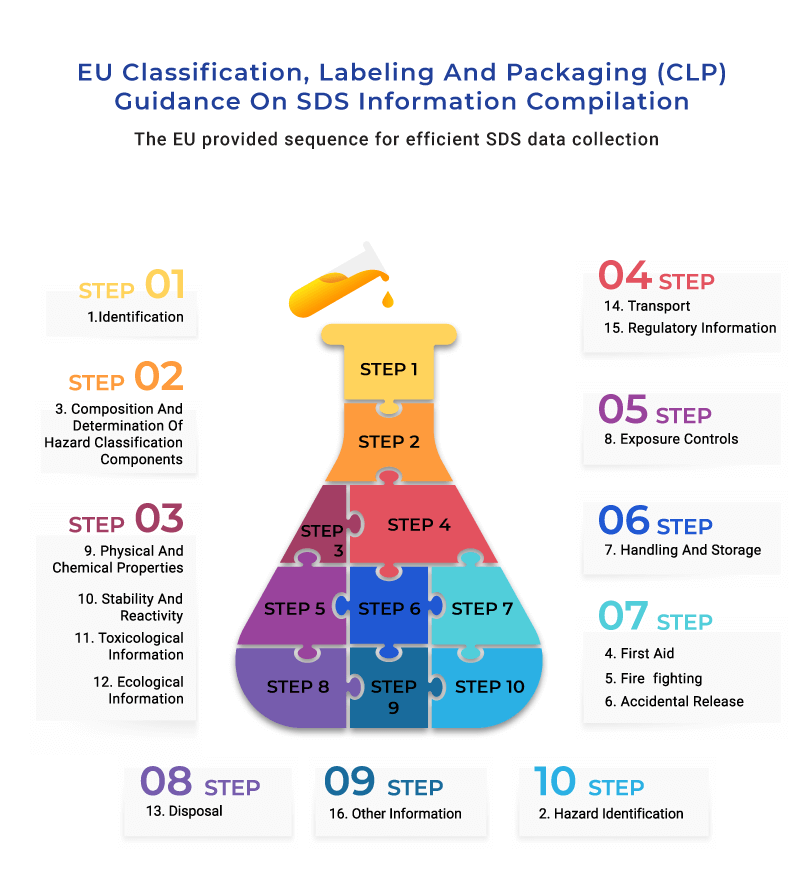
Each section of the SDS requires thorough research and evaluation. For example, determining hazard classification, such as Acute Toxicity, requires many formulas and must be classified based on exposure routes and hazard severity. In contrast, carcinogenicity classification needs evaluation of existing data to put them in one of three categories accurately. ERA has detailed eBooks on various ways of determining these properties.
Revised Requirements for EU Safety Data Sheets - Regulation 2020-878-EU Commission
CLP compliance requires up-to-date knowledge of requirements and changes to ensure accurate SDS. The EU published REGULATION (EU) 2020/878, which defines new content. From December 2022 forward, the EU SDS requests variations in the SDS sections concerning product identification and hazard communications.
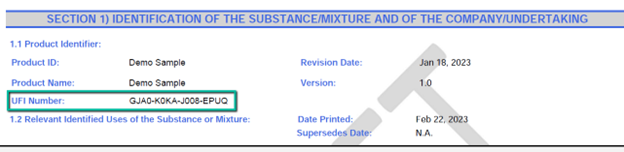
Section 1 has been amended pursuant to REGULATION (EU) 2020/878. According to CLP regulations, each chemical requires a Unique Formula Identifier (UFI), and it should be clearly indicated on the first page of the SDS. If you do not have a readily available UFI, you can generate one at https://ufi.echa.europa.eu/#/create.
Endocrine-disrupting properties of the substance must be clearly indicated throughout multiple sections of the SDS, including Section 2 (Category 2.3: Other hazards), 11 (Subcategory 11.2.1: Endocrine Disrupting Properties) and 12 (Category 12.6: Endocrine Disrupting Properties).
Below are the phrases that you should use if your substance contains endocrine chemicals or no endocrine chemicals.
Section 2
Containing Endocrine chemicals
2.3 Other hazards
The substance(s) below is included on the list established in accordance with Article 59(1) of REACH for having endocrine disrupting properties, or is identified as having endocrine disrupting properties in accordance with the criteria set out in Commission Delegated Regulation (EU) 2017/2100 or Commission Regulation (EU) 2018/605.
No Endocrine chemicals
- Other hazards
The substance(s) is not included in the list established in accordance with Article 59(1) of REACH for having endocrine disrupting properties, or is not identified as having endocrine disrupting properties in accordance with the criteria set out in Commission Delegated Regulation (EU) 2017/2100 or Commission Regulation (EU) 2018/605.
Section 11
Containing Endocrine chemicals
11.2.1 Endocrine disrupting properties
Adverse health effects caused by endocrine disrupting properties : The substance(s) below is included on the list established in accordance with Article 59(1) of REACH for having endocrine disrupting properties, or is identified as having endocrine disrupting properties in accordance with the criteria set out in Commission Delegated Regulation (EU) 2017/2100 or Commission Regulation (EU) 2018/605.
No Endocrine chemicals
11.2.1 Endocrine disrupting properties
Adverse health effects caused by endocrine disrupting properties: No data available
Section 12
Containing Endocrine chemicals
12.6 Endocrine Disrupting Properties
The substance(s) below is included on the list established in accordance with Article 59(1) of REACH for having endocrine disrupting properties, or is identified as having endocrine disrupting properties in accordance with the criteria set out in Commission Delegated Regulation (EU) 2017/2100 or Commission Regulation (EU) 2018/605.
No Endocrine chemicals
12.6 Endocrine Disrupting Properties
The substance(s) is not included in the list established in accordance with Article 59(1) of REACH for having endocrine disrupting properties, or is not identified as having endocrine disrupting properties in accordance with the criteria set out in Commission Delegated Regulation (EU) 2017/2100 or Commission Regulation (EU) 2018/605.
Click the image below to view a fully compliant CLP sample SDS to guide you in creating your own SDS. The sample is generated using the ERA SDS authoring solution.
SDS Authoring, the ERA way
All these details can be challenging to remember when authoring your SDS. According to the American Chemical Society, an individual would require 8 to 16 hours to produce the first draft of a fully compliant SDS without software assistance. At ERA, we believe that no one should have to work this hard on a single product; our tool is expertly built and incorporates the expertise of many chemical experts to provide you with accurate and compliant SDS in a matter of minutes, including the above-mentioned CLP changes.
ERA’s software has been updated to include:
- A to add the UFI number on your SDS.
- The ability to indicate nanoform information
- The functionality to identify whether a chemical classifies as endocrine disruptive or not, and to display the statement under Section 2 (Category 2.3: Other hazards), 11 (Subcategory 11.2: Endocrine Disrupting Properties) and 12 (Category 12.6: Endocrine Disrupting Properties)
Watch a personalized demo below to learn how you can have quick access to fully compliant SDS and in-depth consultation from seasoned experts.
References
- European Commission. (2020). Commission Regulation (EU) 2020/878 of 18 June 2020 amending Annex II to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). J. Eur. Union, 203, 28-58. https://echa.europa.eu/documents/10162/2324906/sds_en.pdf/01c29e23-2cbe-49c0-aca7-72f22e101e20
-
Compilation of Safety Data Sheets - Europa. (2020). Retrieved 23 February 2023, from https://echa.europa.eu/documents/10162/2324906/sds_nutshell_guidance_en.pdf/5d5eff4a-3596-4ba8-a4c8-3311ba4ad07b
- DeMasi, A., Elston, H., & Langerman, N. (2022). Safety Data Sheets: Challenges for Authors, Expectations for End-Users. ACS Chemical Health & Safety. https://pubs.acs.org/doi/abs/10.1021/acs.chas.2c00015

March 13, 2023




Comments